Russian health authorities are on the verge of introducing a breakthrough personalized cancer vaccine Russia has developed, named EnteroMix. Created by the National Medical Research Radiological Centre (NMRRC) in collaboration with leading Russian institutions including the Gamaleya Center, the vaccine represents a major mRNA oncology breakthrough and is now in Phase 1 clinical testing with highly promising results.
Clinical Testing and Regulatory Readiness
At the Eastern Economic Forum (EEF) 2025, Veronika Skvortsova, head of the Federal Medical and Biological Agency (FMBA), confirmed that EnteroMix has completed a full cycle of preclinical studies (2021–2024) and is now undergoing Phase 1 human trials. All documentation has been submitted to the Ministry of Health for approval (RTVI, Pravmir, Haqqin.az).
The preclinical trials, carried out over three years, confirmed safety even after repeated administrations and demonstrated strong antitumor activity, with tumor reduction rates between 60% and 80% depending on tumor type and patient profile.
Based on these results, Phase 1 clinical trials began in December 2024, enrolling 48 patients with advanced, treatment-resistant cancers. Recruitment was completed by February 2025. The trial is scheduled to run until October 2026, with primary goals of assessing safety, tolerability, and pharmacokinetics, while secondary endpoints monitor early signs of tumor response.
Developers and Scientific Leadership
EnteroMix was developed by Russian research organizations including the National Medical Research Center of Radiology and the V.A. Engelhardt Institute of Molecular Biology (IMB RAS).
The project is led by Academician Andrei Kaprin, Chief Oncologist of the Ministry of Health and Director General of the NMRRC, with scientific leadership from virologist Petr Chumakov (IMB RAS). Chumakov continues the pioneering research of his mother, Marina Voroshilova, who first discovered the oncolytic properties of enteroviruses in the 1970s. The name “EnteroMix” reflects its composition: a mixture of enteroviruses designed to selectively target tumors.
Mechanism of Action
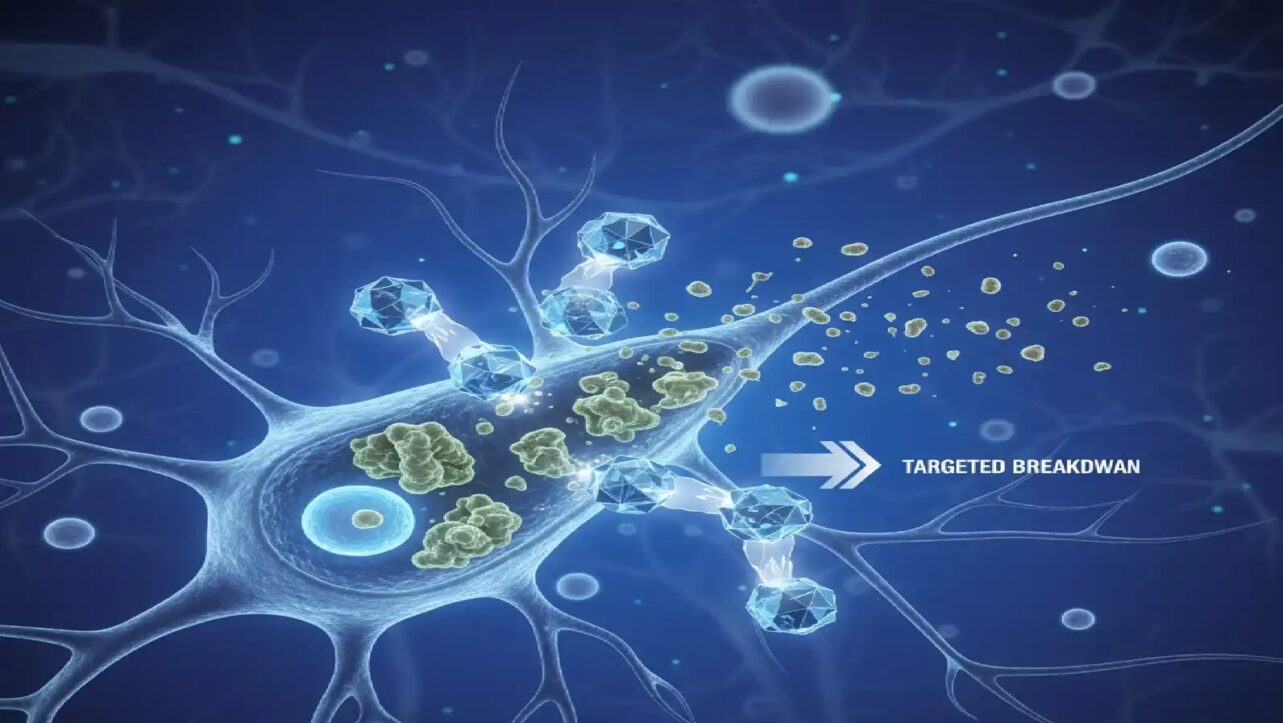
Unlike preventive vaccines, oncovaccines like EnteroMix are used to treat existing tumors. EnteroMix is produced as a frozen viral solution, administered intravenously, directly into tumors, or via cellular carriers.
Its mechanism is based on oncolytic virotherapy:
- The viruses act as a “Trojan horse”, infecting and replicating inside tumor cells, causing their lysis (death).
- This process releases tumor antigens, teaching the immune system to recognize and destroy cancer cells.
- The vaccine therefore exerts a dual function – direct tumor destruction and immune activation, engaging both innate (NK cells, interferons) and adaptive (T lymphocytes) immunity.
By combining four different non-pathogenic enteroviruses, EnteroMix expands its coverage across heterogeneous tumors. Developers note that while some cancers (like melanoma) are highly immunogenic, others respond weakly; the viral mix aims to overcome this challenge (Security Lab, NMRRC).
Types of Cancer Targeted
EnteroMix is intended for solid tumors including:
- Head and neck cancers
- Lung and mediastinal tumors
- Gastrointestinal cancers (colorectal, liver, pancreas, bile ducts)
- Breast cancer
- Genitourinary cancers
- Primary brain tumors (glioblastoma)
- Sarcomas of soft tissue and bone
- Melanomas (cutaneous, mucosal, ocular)
Patients with hematological malignancies (e.g., leukemia, lymphoma) are excluded, as the vaccine’s mechanism targets solid tumor biology.
Clinical Trial Progress and Interim Results
The Phase 1 trial is designed as an open, single-center study at the NMRRC. Using a dose-escalation protocol, patients receive gradually increasing doses to identify safe and effective administration levels.
- Safety: Preliminary reports indicate minimal toxicity, with only mild, expected side effects such as fever or local inflammation.
- Immunostimulation: Early biomarker data show immune activation consistent with the vaccine’s design.
- Efficacy signals: While Phase 1 is not intended to prove efficacy, researchers are monitoring for tumor stabilization or regression.
According to Kaprin, the vaccine has so far shown “very insignificant toxic effect,” making safety its strongest feature at this stage (RIA Crimea, Tochka.by).
Development Timeline
| Stage | Period | Participants | Cancers | Goal | Results |
|---|---|---|---|---|---|
| Preclinical | 2021–2024 | Animal models | Solid tumors | Efficacy, safety | High efficiency, minimal toxicity; tumor regression observed |
| Phase I Clinical | Dec 2024 – Oct 2026 (ongoing) | 48 patients | Advanced, resistant solid tumors | Safety, tolerability, PK | Interim: safe, immunostimulatory, dose escalation in progress |
| Planned Phase II/III | 2026–2027 | Larger cohorts | Focused tumor types | Efficacy, survival | Pending |
If all phases succeed, approval and clinical rollout could occur by 2027, potentially making EnteroMix the first publicly available personalized mRNA cancer vaccine. Russian authorities have indicated it will be offered free of charge to patients through the state healthcare system.
Broader Implications
EnteroMix exemplifies Russia’s biotech innovation, leveraging expertise from the COVID-19 vaccine race to pioneer cancer immunotherapy. Its design builds on decades of Soviet virotherapy research while integrating modern clinical trial rigor.
Alexander Gintsburg, director of the Gamaleya Institute, stressed its uniqueness:
“Every tumor is individual. EnteroMix adapts to the genetic profile of each patient’s cancer, making it a true personalized therapy.”
If successful, EnteroMix could redefine global cancer treatment paradigms, offering patients a novel, immune-driven option where conventional therapies fail (BFM.ru, Business Today, KT Press).