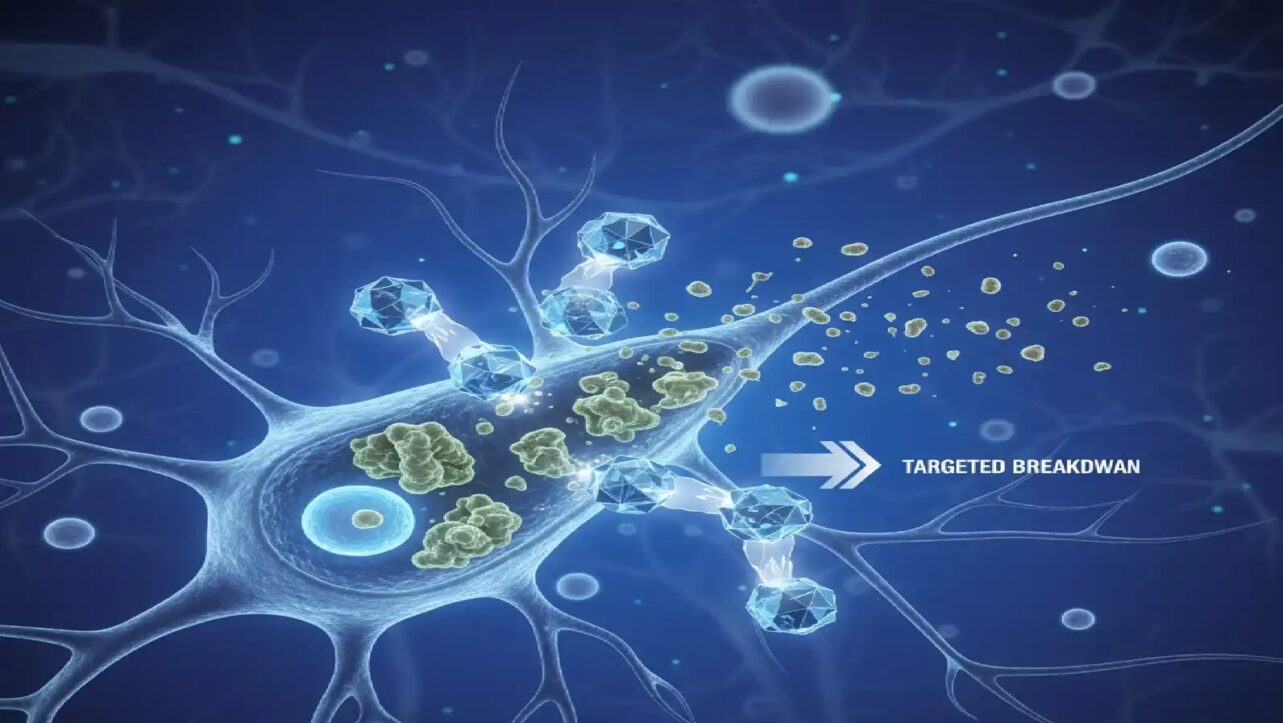
Scientists at the University of Wollongong, Australia have unveiled a revolutionary therapy that removes toxic proteins from nerve cells before they can cause damage—a world-first discovery that could transform treatment for Motor Neurone Disease (MND).
The groundbreaking research, published today in Nature Communications, introduces MisfoldUbL, a designer molecule that targets and eliminates misfolded SOD1 (superoxide dismutase 1) proteins from cells. This proof-of-concept study represents a major advancement in the fight against MND, a devastating disease that gradually destroys motor neurons, leading to muscle weakness, paralysis and death.
The Misfolded Protein Problem
Inherited MND carry SOD1 gene mutations that cause proteins to misfold more frequently. SOD1 is normally an antioxidant enzyme that protects cells from damage, but when it misfolds, the consequences are catastrophic.
“In MND, proteins misfold more frequently and the cell’s degradation systems become overwhelmed and stop working properly,” explained lead researcher Dr. Christen Chisholm from UOW’s Molecular Horizons. “The misfolded protein can then accumulate, forming clumps or ‘aggregates’ and over time, this accumulation damages and eventually kills motor neurons“.
How the Designer Molecule Works
MisfoldUbL acts like a protein recycling tag, developed in collaboration with industry partner ProMIS Neurosciences. The molecule attaches to misfolded SOD1 proteins and directs the cell’s waste-disposal system to break them down before they form toxic clumps.
“We wanted to design a therapy that could help the cells get rid of harmful misfolded SOD1 before it could accumulate into aggregates,” Dr. Chisholm said. “To do this we needed a way to identify the misfolded protein in the sea of cellular proteins. Once identified, we needed a way to feed misfolded SOD1 into the cell’s degradation systems“.
The research team used the biological PROTAC (BioPROTAC) approach, employing an antibody fragment (scFv) that specifically recognizes misfolded SOD1 variants and fuses it to an E3 ligase enzyme that tags proteins for degradation.
Promising Results in Animal Models
Testing in SOD1 G93A mice—a standard model for ALS research—revealed significant protective effects. Expression of MisfoldUbL in neurons delayed disease onset, slowed disease progression, and preserved motor control and function until the end stage of disease.
The therapy resulted in a measurable reduction of insoluble SOD1 in brain tissue at both early-symptomatic and end stages. Most importantly, the treatment protected motor neurons in the ventral lumbar spinal cord and preserved innervated neuromuscular junctions—the critical connections between nerves and muscles.
Male mice showed particularly strong benefits from the therapy, though the physiological reasons underlying sex-specific differences in ALS remain unclear. While the treatment slowed symptom development and preserved motor function, it did not extend overall survival in this mouse model, possibly due to lower expression levels in the spinal cord compared to the brain.
A Legacy of Hope
The project was initially led by the late Professor Justin Yerbury, who received a $1 million FightMND Drug Development Grant in 2020. Professor Yerbury, a carrier of the SOD1 gene mutation himself, dedicated his career to understanding the protein’s role in MND development before losing his own battle with the disease in 2023.
Dr. Chisholm, a former high school science teacher and mother of three, was inspired by Professor Yerbury to change careers and join his lab following his MND diagnosis. Early in her PhD, she took the helm of this project after his death.
“This research is the result of years of dedicated effort by many amazing scientists, all inspired by Justin and driven to advancing our understanding of MND and how to treat it,” Dr. Chisholm said. “I am especially honoured that Justin entrusted his idea to me to develop and I’m so proud and grateful to all the people who helped me bring his idea to fruition“.
Next Steps and Future Potential
The researchers acknowledge that broader and stronger expression of MisfoldUbL may be required to combat the disease fully, particularly in the spinal cord where pathology is critical. An important next step involves investigating this strategy in patient-derived cell lines that may better capture endogenous expression levels rather than the overexpressed mutant SOD1 in mouse models.
This research was funded by donations through FightMND, which announced nearly $22.9 million in MND research and care investments for 2025. The charity’s 2025 funding includes $21.5 million into research and infrastructure projects aimed at finding effective treatments and a cure.
“This incredibly important research was funded by donations through FightMND and wouldn’t have happened without the support of people who contribute their hard-earned money in the hope that we can make progress in the fight against this devastating disease,” Dr. Chisholm emphasized.