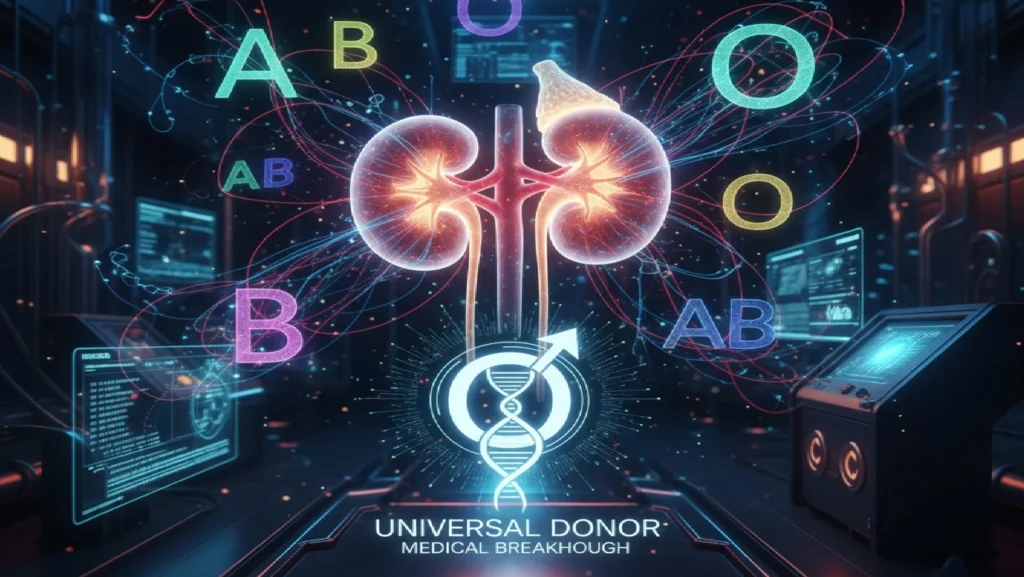
In a groundbreaking medical first, scientists have successfully converted a blood type A kidney into universal type O and transplanted it into a human, marking a potential turning point in addressing the global organ shortage crisis. The pioneering procedure, published October 2, 2025, in Nature Biomedical Engineering, demonstrates that changing an organ’s blood type is not only possible but could dramatically improve access to transplants for patients dying on waiting lists.
The achievement represents more than a decade of research by teams from the University of British Columbia (UBC) in Canada and West China Hospital in Chengdu, China. For the first time, the blood type barrier—one of the most fundamental obstacles in organ transplantation—has been overcome in a human body.
The Blood Type Problem Killing Patients on Waiting Lists
More than 100,000 Americans currently await kidney transplants, with similar crises in Canada (3,500 waiting), India (175,000-200,000 need transplants annually but only 10,000-12,000 receive them), and countries worldwide.
Currently, organs from deceased donors can only be transplanted to recipients with compatible blood types. The recipient’s immune system produces antibodies that attack and destroy donated organs if the donor and recipient have different antigens— that define blood types A, B, AB, or O.
Type O patients suffer most severely. Comprising more than half of kidney transplant waiting lists, type O patients can only receive organs from type O donors. However, because type O kidneys lack A or B antigens, they’re universally compatible and frequently allocated to patients with other blood types. The result: type O patients wait 2-4 years longer than others, and many die waiting.
“Anyone can receive type O organs because they don’t have A or B antigens,” explains the research team. “But type O patients are stuck waiting for the limited supply of type O donors while watching compatible organs go to others“.
The Enzyme Solution: Changing the Organ, Not the Patient
Traditional methods for overcoming blood type incompatibility require days of intensive treatment to strip antibodies and suppress the recipient’s immune system—a risky, demanding process that only works with living donors where timing can be controlled.
The UBC-developed enzyme approach is revolutionary because it changes the organ rather than the patient, potentially enabling faster transplants with fewer complications. Most importantly, it could unlock blood type-mismatched organs from deceased donors—where every hour determines whether a patient lives or dies.
How the Enzyme Technology Works
Researchers used specialized enzymes discovered by the UBC team in 2019 to remove type A antigens from the donor kidney. The process takes just two hours using organ preservation machines:
- Enzyme perfusion – The kidney is treated with highly selective enzymes through preservation equipment
- Antigen removal – Enzymes act as “molecular scissors,” cutting off the sugar molecules that mark the organ as type A
- Type O reveal – Removing A antigens reveals the universal type O underneath
- Transplantation – The converted kidney is transplanted using standard protocols
“It’s like removing the red paint from a car and uncovering the neutral primer,” said Dr. Stephen Withers, UBC professor emeritus of chemistry who co-led the enzyme development. “Once that’s done, the immune system no longer sees the organ as foreign“.
The First Human Transplant: What Happened
In the pioneering first-in-human experiment, the enzyme-converted kidney was transplanted into a 68-year-old brain-dead man in Chongqing, China, with family consent. This approach allowed researchers to observe the immune response in a real human body without risking a living patient’s life.
The Results: Promising But With Challenges
Days 1-2: The kidney functioned without signs of hyperacute rejection—the rapid, devastating immune reaction that can destroy an incompatible organ within minutes. This was the critical proof that the enzyme treatment successfully prevented immediate rejection.
Day 3: Some blood-type antigens began to reappear on the kidney’s surface, triggering a mild immune reaction. However, the damage was far less severe than in typical blood type mismatches, and researchers observed signs the body was beginning to tolerate the organ.
Day 6: The kidney continued producing urine through at least the sixth day of observation.
“This is the first time we’ve seen this play out in a human model,” said Dr. Withers. “It gives us invaluable insight into how to improve long-term outcomes“.
The lack of immediate hyperacute rejection represents the key success—demonstrating that the enzyme treatment works in principle. The antigen reappearance on day three shows researchers what challenge to solve next.
A Decade of Discovery: From Blood to Organs
The breakthrough is the culmination of research that began in the early 2010s when Dr. Withers and colleague Dr. Jayachandran Kizhakkedathu, a UBC professor in pathology and laboratory medicine, started working on making universal donor blood by stripping away blood-type-defining sugars.
2019: The team discovered two highly efficient enzymes that remove the sugar defining type A blood, effectively converting it to type O. “These enzymes are highly active, highly selective, and work at very low concentrations,” said Dr. Kizhakkedathu. “That made the whole concept feasible“.
2022: The team showed that a type A lung could be converted to type O, though it wasn’t transplanted into a person.
2023: While Dr. Kizhakkedathu was on an overseas trip, collaborators in China showed him data from the first successful human transplant. “They had converted a human kidney and transplanted it into a brain-dead recipient. It was working beautifully,” he recalled. He stayed up late to call Dr. Withers first thing in the morning in British Columbia. “I was so thrilled. It was a dream moment“.
October 2025: Results published in Nature Biomedical Engineering, marking the first time a converted organ has been tested in a human immune system.
Expert Reaction: “Groundbreaking”
Dr. Natasha Rogers, a transplant clinician at Westmead Hospital in Sydney, Australia, called the results “groundbreaking,” noting they could improve access to donor organs and reduce transplant waiting lists.
“If the blood type of the organ was no longer a barrier for transplantation, physicians could focus on things such as matching other antigens unrelated to blood type, which are important in terms of how long a transplant will last,” Rogers explained.
These other antigens Rogers refers to are HLA (Human Leukocyte Antigens)—the tissue compatibility markers that must still be matched even with blood type-compatible organs. The enzyme breakthrough solves the blood type problem but not the HLA matching requirement, meaning recipients still need immunosuppressive drugs and compatible tissue types.
The Indian Context: Massive Potential Impact
India faces one of the world’s most severe organ shortage crises. Despite a population of 1.4 billion, the country performs only 10,000-12,000 kidney transplants annually against an estimated need of 175,000-200,000.
How this breakthrough could help India:
- Maximize every donor kidney – Each donated organ could potentially be used regardless of blood type
- Help type O patients – The largest group on waiting lists would gain access to A, B, and AB donor kidneys
- Reduce deceased donor dependency – More efficient use of available organs in a country with limited donation rates
- Improve tier-2/3 city access – Smaller hospitals with limited donor pools could use more available organs
However, HLA matching facilities and immunosuppression infrastructure would still be required.
What This Doesn’t Solve: The HLA Reality
While media coverage has focused on “universal donor organs,” it’s crucial to understand the limitation: this technique only addresses ABO blood type antigens, not HLA tissue compatibility.
What patients still need:
- HLA compatibility testing and matching
- Immunosuppressive medications to prevent HLA-mediated rejection
- All standard transplant monitoring and care
- Protection against acute and chronic rejection
The breakthrough expands the donor pool by eliminating blood type restrictions but doesn’t create truly “universal” organs that anyone can receive without further compatibility checks and immunosuppression. Even with blood type conversion, recipients face lifelong immunosuppression due to HLA mismatches. While 3D bioprinted organs from patients’ own cells could theoretically eliminate rejection, enzyme conversion provides a lifelong bridge solution.
The Challenge Ahead: Preventing Antigen Regeneration
The reappearance of type A antigens on day three is the key problem researchers must now solve. Scientists believe antigens may be regenerated by the recipient’s cells or could come from blood vessel cells deeper in the kidney that weren’t fully exposed to enzymes during the two-hour treatment.
Current research priorities:
- Preventing permanent antigen regeneration
- Extending organ function beyond days to years
- Scaling the process for widespread clinical use
- Extending the technique to hearts, lungs, livers, and other organs
Global Implications: Who Benefits Most?
Type O patients (over 50% of waiting lists) would gain the most, potentially receiving kidneys from type A, B, or AB donors after conversion.
Organ allocation systems could become more efficient, with fewer viable organs discarded due to blood type mismatch alone.
Deceased donor transplants could happen faster since time-consuming recipient immune preparation wouldn’t be needed for blood type issues.
Worldwide impact: Hundreds of thousands on transplant waiting lists in the US, India, China, and globally could benefit.
What’s Next: The Path to Clinical Trials
While the results are promising, significant work remains before this becomes standard practice:
Immediate research goals:
- Additional decedent studies to evaluate longer-term outcomes
- Solving the antigen regeneration problem
- Refining enzyme treatment protocols
Clinical development:
- First-in-human trials with living recipients
- Safety and efficacy studies
- Regulatory approval processes
- Extension to other organs beyond kidneys
The decedent transplant model used in this study is recognized by the FDA as a viable pathway toward clinical trials, providing regulatory clarity for advancement.
The Science: Blood Type Antigens Explained
Blood types (A, B, AB, O) are determined by specific sugar molecules called antigens on the surface of red blood cells and organ tissues:
Type A: Has A antigens
Type B: Has B antigens
Type AB: Has both A and B antigens
Type O: Has neither A nor B antigens (universal donor for blood type)
When a patient receives an organ with incompatible antigens, their immune system recognizes these foreign markers and attacks, causing rejection. The UBC enzymes work by cleaving off these sugar “nametags,” revealing the type O surface beneath.
Why This Matters: The Shortage Crisis
“The shortage of donated organs for transplantation has resulted in lengthy waiting lists and consequent deaths,” the research team wrote. “Even when organs are available, substantial challenges exist with the equality of organ allocation“.
“While transplantation of engineered animal organs offers some hope for the future, this remains many years away from general practice owing to the recent failure of engineered pig hearts and kidneys in humans, meaning that more efficient use of the available human organ pool is needed,” they added.
This enzyme technology represents exactly that—a way to use existing human donor organs more efficiently by eliminating blood type as a barrier to transplantation.
The Bottom Line
The successful conversion and transplantation of a blood type A kidney to type O represents a significant advance in addressing the organ shortage crisis. While the technique currently faces challenges—particularly preventing antigen regeneration—it has demonstrated proof of concept in a human body.
What this breakthrough achieves:
- Eliminates the blood type barrier in organ transplantation
- Could dramatically expand donor pools, especially for type O patients
- Works with deceased donor organs where timing is critical
- Enables faster transplants without days of recipient immune preparation
What it doesn’t solve:
- HLA tissue compatibility still requires matching
- Recipients still need immunosuppressive drugs
- Organs aren’t truly “universal” in the complete sense
- Standard transplant protocols and monitoring remain necessary
For the millions worldwide waiting for kidney transplants—including the 175,000-200,000 in India and over 90,000 in the United States—this breakthrough offers genuine hope. If researchers can refine the technique to ensure permanent conversion, blood type could cease to be a barrier to transplantation, saving countless lives by ensuring more patients can find compatible organs from the available donor pool.
The enzyme technology isn’t a complete solution to transplant compatibility, but it’s a major step forward that could help thousands of patients who previously had limited options due to their blood type.