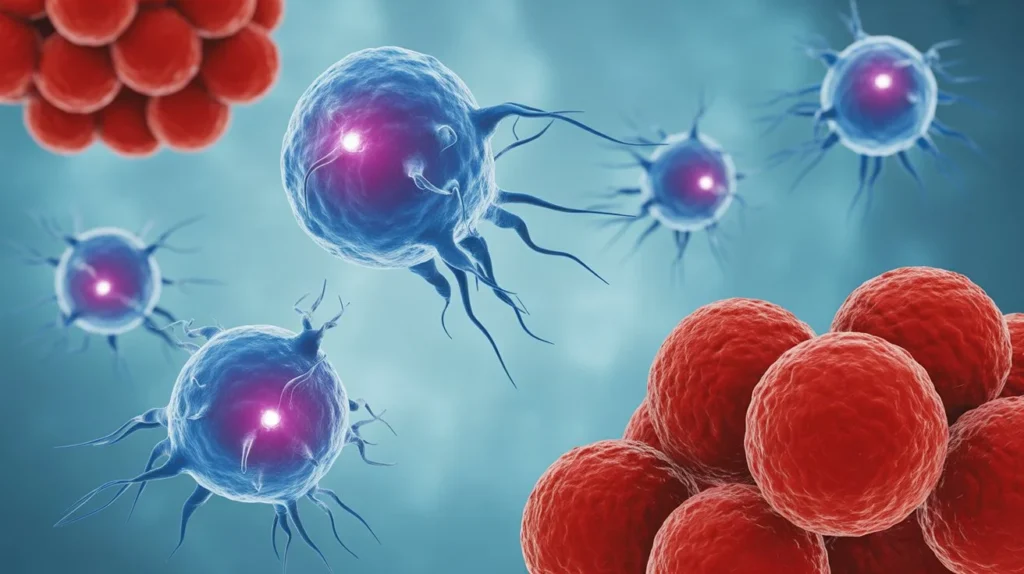
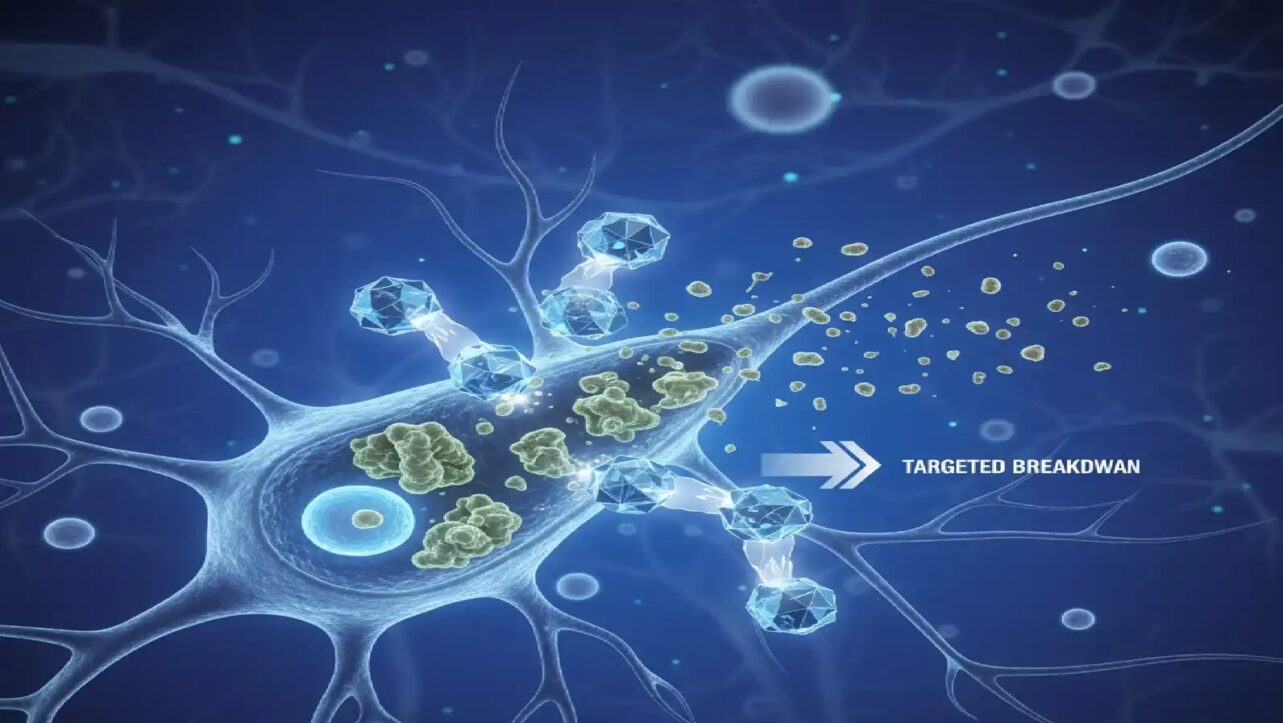
Scientists at MIT and Harvard Medical School have engineered advanced immune cells that can destroy cancer while avoiding attack from the body’s own immune defenses, potentially revolutionizing cancer treatment with faster, safer, and more accessible therapies.
The breakthrough, published in Nature Communications on October 8, 2025, addresses one of the biggest challenges in cancer immunotherapy: immune rejection. The new CAR-NK (chimeric antigen receptor natural killer) cells could be manufactured as “off-the-shelf” treatments ready immediately after diagnosis, rather than waiting weeks for personalized therapies.
One-Step Engineering Overcomes Major Barrier
Traditional cell-based cancer therapies face a critical limitation—the patient’s immune system often rejects the engineered cells, weakening their effectiveness. CAR-T cell therapy, which has shown remarkable success against blood cancers, typically requires several weeks to manufacture from each patient’s own cells and costs hundreds of thousands of dollars.
“This enables us to do one-step engineering of CAR-NK cells that can avoid rejection by host T cells and other immune cells. And, they kill cancer cells better and they’re safer,” says Jianzhu Chen, MIT professor of biology, member of the Koch Institute for Integrative Cancer Research, and senior author of the study.
The MIT-Harvard team developed a genetic modification that allows donor-derived NK cells to hide from the patient’s immune system. This single-step process makes it possible to create ready-to-use cells that can be administered to patients immediately after diagnosis, eliminating the weeks-long wait required by traditional methods.
Natural Killer Cells: The Body’s Sentinels
Natural killer (NK) cells are a crucial part of the immune system with the innate ability to destroy abnormal or infected cells without prior “training” to recognize threats. Unlike T cells, which must be specifically primed to identify targets, NK cells constantly patrol the body searching for cells that display signs of danger.
CAR-NK cells offer several advantages over CAR-T cells:
- No cytokine release syndrome (CRS): CAR-T therapy often causes CRS, a serious side effect that can damage multiple organs
- No neurotoxicity: CAR-NK cells avoid the neurological complications sometimes seen with CAR-T therapy
- No graft-versus-host disease (GvHD): CAR-NK cells can be transplanted without alloreactivity, making them true “off-the-shelf” products
- No HLA compatibility required: Patients don’t need tissue matching, expanding treatment availability
Remarkable Results in Humanized Mice
In tests using mice with humanized immune systems—mice engineered to have immune cells similar to humans—the newly engineered CAR-NK cells demonstrated extraordinary results:
Treatment with the novel CAR-NK cells resulted in sustained cell persistence for at least three weeks, coupled with robust tumor clearance. The modified cells successfully destroyed most cancer cells while avoiding attack from the host’s own immune defenses.
In stark contrast, mice that received unmodified NK cells or standard CAR-NK cells lost the therapeutic cells within two weeks, and their tumors continued to spread.
The team’s construct targets CD19, a protein found on many cancerous B cells, making it particularly effective against lymphoma—a type of blood cancer affecting the lymphatic system. In the United States alone, lymphoma affects approximately 90,000 people each year and causes 20,000 deaths.
From Concept to Clinical Applications
The study’s lead author, Fuguo Liu, a postdoctoral researcher at MIT’s Koch Institute and research fellow at Dana-Farber Cancer Institute, helped drive the innovation from concept through successful mouse trials. Rizwan Romee, associate professor of medicine at Harvard Medical School and Dana-Farber Cancer Institute, served as co-senior author.
The research team used selective HLA (human leukocyte antigen) knockdown and PD-L1 expression to prevent immune rejection while maintaining the cells’ cancer-killing abilities. This approach represents a major advance in allogeneic cellular immunotherapy—using donor cells rather than the patient’s own cells.
Beyond Cancer: Treating Autoimmune Disease
The researchers aren’t stopping at cancer applications. The team is now collaborating with a biotechnology company to explore CAR-NK cells as a treatment for lupus, an autoimmune disorder where the immune system attacks healthy tissues and organs.
Lupus affects over 1.5 million Americans and causes the immune system to mistakenly target healthy tissues throughout the body. If successful, this application could mark a new chapter in immunotherapy—one where engineered cells not only fight disease but restore balance to misfiring immune systems.
Addressing Previous Limitations
Earlier research had shown that while CAR-NK cells offered safety advantages, they often underperformed compared to CAR-T cells in direct cancer-killing ability. Studies found that CAR-NK cells were only effective at high doses and their activity decreased rapidly over time compared to CAR-T cells.
The MIT-Harvard breakthrough addresses these limitations through strategic genetic modifications that enhance both the cells’ survival and their tumor-killing power. The genetic modification used is relatively simple, which could make large-scale manufacturing feasible for widespread clinical use.
Clinical Trials on the Horizon
CAR-NK cells are currently being tested in clinical trials for lymphoma and several other cancers. The team is now preparing for human clinical trials with their enhanced version, hoping to bring these “stealthy immune assassins” into hospitals and oncology wards.
The construct could also be adapted for use against other cancer types beyond lymphoma, potentially expanding treatment options for patients with various malignancies.
Global Implications for Cancer Care
The development of off-the-shelf CAR-NK therapy could democratize access to advanced cancer immunotherapy. Current CAR-T therapies, while effective, remain prohibitively expensive and time-consuming for many patients, particularly in developing nations.
By creating pre-manufactured treatments that don’t require tissue matching and can be administered immediately, this technology could bring cutting-edge cancer therapy to patients who currently lack access to such innovations. The reduced risk of serious side effects also makes the treatment safer for a broader patient population, including older adults who may not tolerate aggressive therapies.
The research represents a convergence of immune engineering, molecular biology, and precision medicine—disciplines that together are reshaping how humanity fights cancer.