Scientists at the University of Washington have engineered therapeutic proteins that can autonomously make decisions inside the human body using Boolean logic gates—the same computational principles that power computers. Published October 9, 2025, in Nature Chemical Biology, the breakthrough represents a paradigm shift in targeted drug delivery, potentially transforming how doctors treat cancer, autoimmune diseases, and other complex conditions.
Revolutionary Technology Reduces Side Effects, Increases Treatment Precision
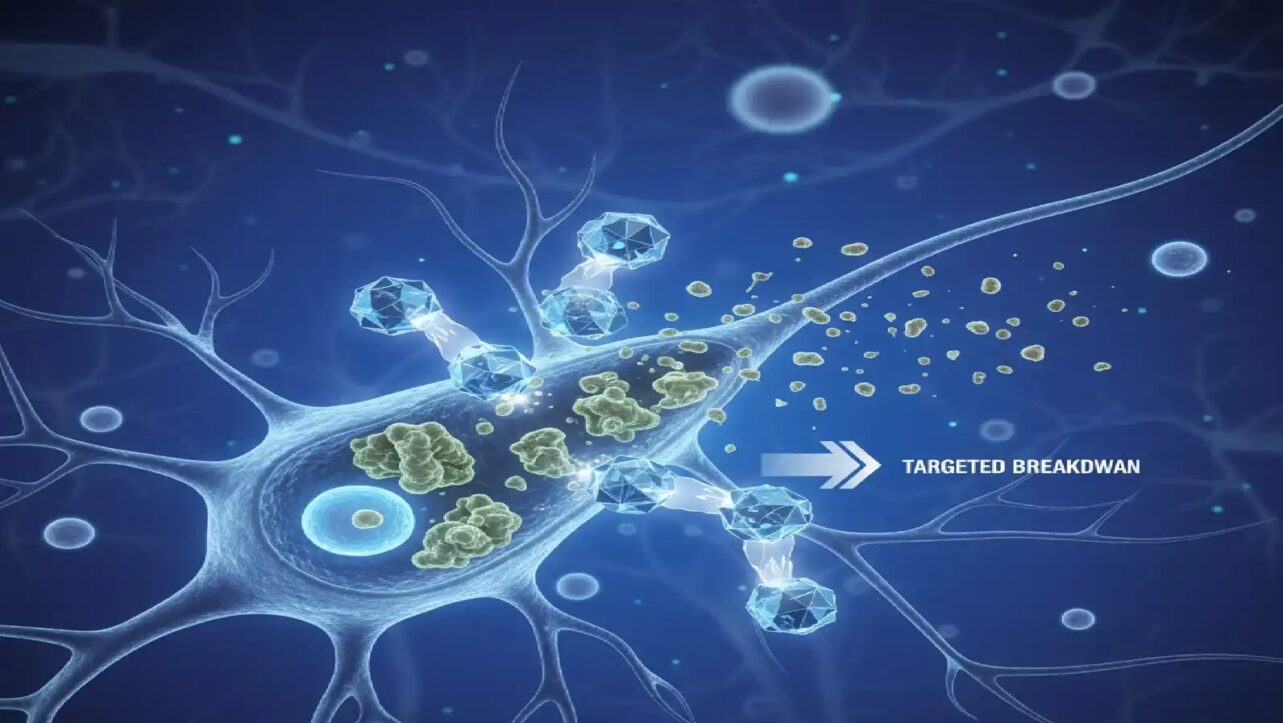
The research team, led by Professor Cole DeForest from UW’s Department of Chemical Engineering and Bioengineering, designed proteins with “smart tail” structures that fold into preprogrammed shapes based on environmental cues like enzyme presence, pH levels, or cancer-specific biomarkers. These molecular decision-makers release therapeutic cargo only when specific combinations of conditions are met—similar to how a computer executes commands when certain criteria are satisfied.
“We’ve now finally figured out how to produce these systems faster, at scale, and with dramatically enhanced logical complexity,” said DeForest. “We are excited about how these will lead to more sophisticated and scalable disease-honing therapies.”
Traditional targeted therapies identify single biomarkers, but this often leads to off-target effects because many biomarkers exist in multiple body locations. The UW team’s innovation overcomes this limitation by requiring multiple biomarkers to be present simultaneously—drastically improving specificity.
How Boolean Logic Gates Work Inside the Body
The proteins utilize three basic logic operations familiar to computer programmers: AND, OR, and YES gates. In an AND gate configuration, therapeutic cargo releases only when two or more specific biomarkers are detected together. For example, a cancer-targeting protein might require both a tumor-associated enzyme and an acidic pH level—conditions that coexist primarily in cancerous tissues—before activating.
OR gates release therapy if either of two biomarkers is present, while YES gates respond to a single cue. By combining these basic gates, researchers created complex “circuits” capable of responding to up to five different biomarkers simultaneously.
“For example, if we linked a therapeutic cargo to a material via two degradable groups connected in series—that is, each after the other—it would be released if either group was degraded, acting as an OR gate,” DeForest explained. “When the degradable groups were instead connected in parallel, both groups had to be degraded for cargo release, functioning as an AND gate.“
Manufacturing Breakthrough Cuts Production Time from Months to Weeks
Perhaps equally revolutionary is the manufacturing process. Previous iterations of logic-responsive biomaterials required laborious manual synthesis through traditional organic chemistry, taking months to produce just a few milligrams. The new approach harnesses synthetic biology techniques, using living cells as protein factories.
Researchers design custom DNA blueprints for new proteins, insert the DNA into bacteria or other host cells, and collect the properly structured proteins directly from the cells. This streamlined process takes just weeks from design to product—a “complete game changer,” according to DeForest.
“Using the old process, it would take months to synthesize just a few milligrams of each of these materials. Now it takes us a couple of weeks to go from construct design to product,” DeForest said.
Multiple Therapies, One Delivery System
In proof-of-concept experiments, the team demonstrated unprecedented versatility. They loaded a single carrier material—such as hydrogels, tiny beads, or living cells—with three different programmable proteins, each designed to deliver unique therapeutic cargo based on different sets of environmental cues.
“You can create delayed and independent delivery of many different components in one treatment,” said co-first author Murial Ross, a UW doctoral student in bioengineering. “And I think we could create much, much larger logical circuits that a protein can be responsive to. We’re at the point now that the technology is outpacing what we’ve seriously considered in terms of applications, which is a great place to be.“
Cancer Treatment Expected as First Clinical Application
DeForest anticipates cancer therapies will be the first practical applications, though the technology holds promise for numerous medical conditions. The research team is now searching for additional biomarkers that proteins could target and seeking collaborations with labs at UW and beyond to build real-world therapies.
Beyond drug delivery, the platform could enable diagnostic tools that detect complex biomarker patterns in blood samples or manufacture therapeutic proteins directly within individual cells.
“The dream is to be able to pick any arbitrary location inside of the body—down to individual cells—and program a material to go and act there,” DeForest said. “That’s a tall order, but with these technologies we’re getting closer. With the right combination of biomarkers, these materials will just get more and more precise.”
Global Implications for Precision Medicine
This development aligns with the broader shift toward precision medicine—treatments tailored to individual patients and disease characteristics. For patients in India and the United States facing limited access to cutting-edge cancer therapies, scalable production methods could make advanced treatments more affordable and widely available.
The research was funded by the National Science Foundation and the National Institutes of Health. Co-authors include Annabella Li, a former UW undergraduate student of chemical engineering; Shivani Kottantharayil, a UW undergraduate student of bioengineering; and Jack Hoye, a UW doctoral student of chemical engineering.
Sources and Further Reading
This article is based on research news published by the University of Washington on October 9, 2025. The original study was published in Nature Chemical Biology by Professor Cole DeForest and colleagues.