In a groundbreaking development announced on October 15, 2025, Google DeepMind’s artificial intelligence has discovered a novel drug combination that can make “cold” tumors visible to the immune system, potentially revolutionizing cancer immunotherapy for millions of patients worldwide.
The AI model, called Cell2Sentence-Scale 27B (C2S-Scale 27B), identified that combining the drug silmitasertib with low-dose interferon increases the immune system’s ability to recognize cancer cells by approximately 50 percent—a discovery validated through laboratory experiments at Yale University, with a preprint published on BioRxiv.
The Cold Tumor Challenge
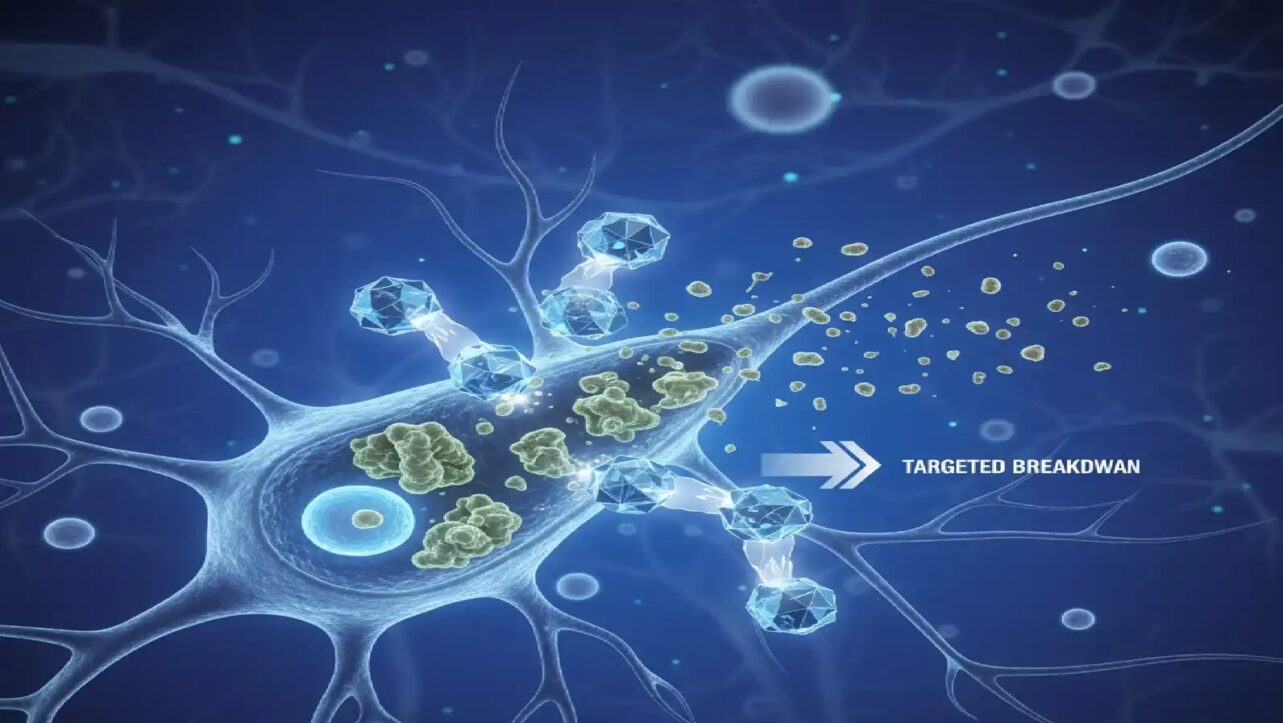
Up to 80 percent of solid tumors are classified as “cold,” meaning they remain invisible to the immune system and resist standard immunotherapy treatments. These tumors have very few immune cells and weak antigen signals, making them nearly undetectable to the body’s natural defenses.
‘What made this prediction particularly thrilling was its originality,’ Google stated in its October 15 announcement. ‘Although CK2 has been implicated in many cellular functions, including as a modulator of the immune system, inhibiting CK2 via silmitasertib has not been reported in the literature to explicitly enhance MHC-I expression or antigen presentation. This highlights that the model was generating a new, testable hypothesis, and not just repeating known facts.’
Google CEO Sundar Pichai called the achievement “An exciting milestone for AI in science:,” emphasizing that this represents one of the first times AI has generated and experimentally validated a completely novel biological hypothesis.
An exciting milestone for AI in science: Our C2S-Scale 27B foundation model, built with @Yale and based on Gemma, generated a novel hypothesis about cancer cellular behavior, which scientists experimentally validated in living cells.
— Sundar Pichai (@sundarpichai) October 15, 2025
With more preclinical and clinical tests,…
How the AI Model Works
The C2S-Scale 27B is a 27-billion-parameter foundation model built on Google’s open-source Gemma AI family, designed to interpret the “language of cells” at a single-cell level. Unlike traditional drug discovery methods that rely on known biological pathways, this AI model can reason through complex biological contexts and identify synergistic effects that humans might miss.
To find the breakthrough combination, the AI analyzed patient tumor data and simulated the effects of over 4,000 drug candidates under two distinct scenarios: one with active immune signaling and another without. The model predicted that silmitasertib (CX-4945), a CK2 kinase inhibitor, would significantly enhance antigen presentation—but only when combined with low-dose interferon in an “immune-positive” environment.
Yale University researchers then validated this prediction in human neuroendocrine cell models. Neither treatment worked alone—silmitasertib produced no change by itself, and low-dose interferon had only modest effects. However, combining the two resulted in a 50 percent increase in antigen presentation, effectively turning the cancer cells from “invisible” to “visible” to immune defenses.
Context-Dependent Reasoning
Researchers emphasized that this context-dependent reasoning represents a new capability acquired by the AI due to its immense size and complexity. Smaller models could not achieve this level of sophisticated biological prediction.
The AI’s virtual test involved simulating the effects of over 4,000 existing drugs on real patient tumor samples and isolated cells without immune context. The model was specifically tasked with predicting which drugs would work only in patient-relevant conditions.
The identification of silmitasertib was particularly surprising because this drug “has not been reported in the literature to explicitly enhance MHC-I expression or antigen presentation,” confirming that the AI generated an entirely new idea rather than summarizing existing knowledge.
Laboratory Validation
When researchers tested the hypothesis in the laboratory using human neuroendocrine cells, results confirmed the AI’s prediction. While silmitasertib or low-dose interferon alone had little effect, treating cells with the combination of both produced powerful amplification.
Google reported that the two drugs together resulted in a roughly 50 percent increase in antigen presentation, dramatically improving the tumor’s visibility to the immune system.
What Happens Next
Yale University researchers are now investigating the exact mechanisms behind the AI’s discovery and testing other predictions generated by the C2S-Scale 27B model across various immune contexts. Researchers worldwide can now access the model through Hugging Face and explore its code on GitHub, potentially building on this discovery to find more treatments.
However, experts caution that these findings require peer review and extensive clinical validation before any therapeutic applications can be pursued. The pathway from laboratory discovery to approved cancer treatment typically requires years of preclinical studies, multiple phases of clinical trials, and regulatory approvals.
The Future of AI in Medicine
The success of C2S-Scale 27B represents a paradigm shift in biological research—moving from traditional trial-and-error methods to AI-driven hypothesis generation at unprecedented speed. “The true potential of scaling resides in the generation of new ideas and the discovery of the unknown,” DeepMind researchers stated.
This development could accelerate drug discovery for cold tumors, which include many of the most challenging cancers such as pancreatic cancer, certain breast cancers, and various solid tumors that resist conventional immunotherapy.
The open-source availability of the C2S-Scale 27B model allows researchers worldwide to build upon this discovery and accelerate the development of new cancer therapies. This cancer discovery is part of Google’s broader MedGemma AI initiative in healthcare, which includes open-access models powering various medical research breakthroughs. Learn more about how Google’s MedGemma models are transforming medicine.
As this technology continues to evolve and more AI-generated hypotheses enter clinical trials, the convergence of open-source AI and collaborative medical research may finally unlock treatments for cancers that have long evaded our best efforts.